What Is the Melting Point of Water at 5000 Meters
Pressure additionally alters the meltingfreezing point. Use the Gizmo to find the freezing meting and boiling points of water at 5000 meters 16404 feet.

Crazy Eddie S Motie News Burning All Fossil Fuels Could Melt Antarctica Ice Melting Ap Human Geography Digital Cartography
Why is there a slight decrease in the melting point of.

. The normal melting point and boiling point of water at 1 atm are 0C and 100C respectively. 32 ºF 0ºC Melting point. Freezing point depression is a colligative property meaning that the effect is observed of pure water at 25C and 1 atm pressure.
At the boiling point the vapor pressure of a liquid equals the external pressure. How did altitude affect the freezing melting and boiling points of water. The presence of a solute lowers the freezing point of any solvent.
Water is completely filled in a rectangular open tank of size 3m x 2m x1m as shown in the. Water usually expands when it freezes. Answer 1 of 2.
This effect is called freezing -. The melting point and freezing point of water ideally are the same especially if there are gas bubbles in water but if the water is free of nucleating points water can supercool all the way down to 42 C 436 F 231 K before freezing. So 5 ft or 5000 ft or 5000 meter it is still 0C.
Use the Gizmo to find the freezing meting and boiling points of water at 5000 meters 16404 feet. Another method to transform the meltingfreezing and boiling point of water is to include salt or any type of substance that will dissolve in water. The incredible press at the basic of special glaciers melts the ice cream at temperatures a couple of degrees Celsius below zero.
What is the freezing point of water at 5000 meters altitude. 4 rows But melting pointfreeze point stays stable un-affected by air-pressure. Write these values below.
Try to explain these results based on the fact that air pressure decreases with altitude. How do solutes affect the freezing point of water. 32 ºF 0ºC Boiling point.
The higher the quantity of liquified. So 5 ft or 5000 ft. May 31 2017 Its downright dry outside when the dew point is at or below the freezing point.
Water 5000 meters altitude Asked Leoncio Bartomeus Last Updated 18th March 2020 Category food and drink cooking 48 7477 Views Votes But melting point freeze point stays stable affected air. But melting pointfreeze point stays stable un-affected by air-pressure. The melting point and temperature of frozen waterice fall slightly when pressure increases.
How did altitude affect the freezing melting and boiling points of water. Write these values below. So first lets give an answer to this question.
What is the freezing point of water at 5000 meters altitude. So in some cases the melting point of water is considerably higher than its freezing point. Ice is formed by freezing liquid water.
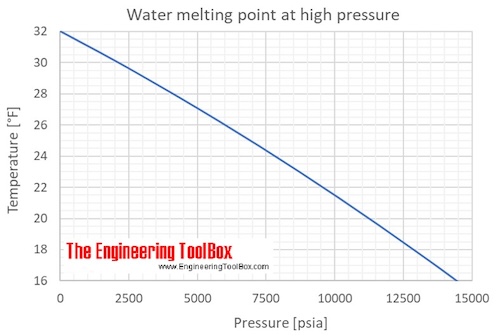
Ice And Water Melting Points Vs Pressure
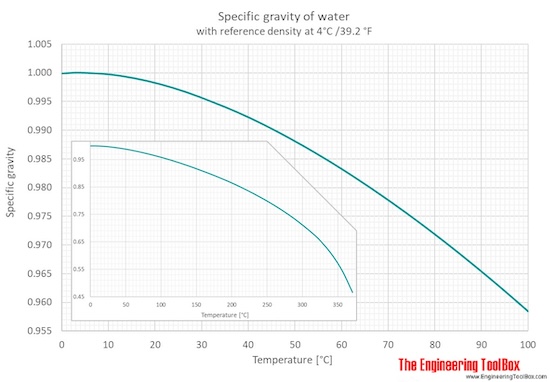
Water Specific Gravity Vs Temperature
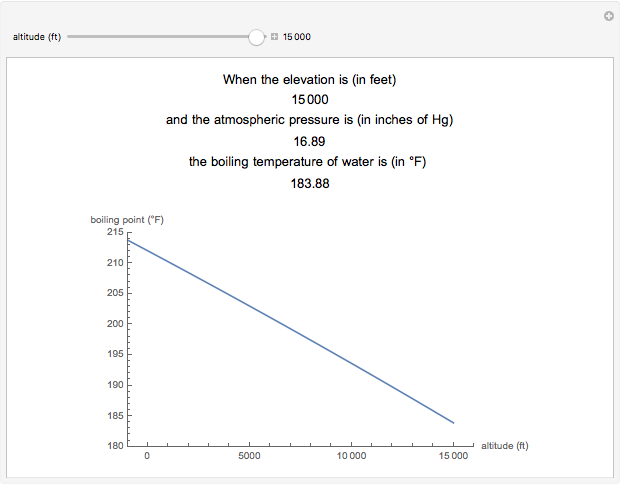
Boiling Point Of Water As A Function Of Altitude Wolfram Demonstrations Project
Comments
Post a Comment